Top 10 best Unicorns In Iceland

Top 10 Unicorns In Iceland
Unicorns: Last week, Iceland Innovation Week, a festival of Icelandic startups, ended in Reykjavik. TFN was on hand to witness the program. In addition to startup work, there were hackathons, exhibits, conferences, and talks about sustainability and invention focusing on climate change.
Transition Labs, an Icelandic climate initiative, was announced at the conference by entrepreneur David Helgason. An Iceland-based climate initiative aims to rapidly deploy its solutions and scale them quickly while partnering with the world’s most impactful climate projects.
Business Iceland also announced the opening of Reykjavik Science City at the same time. A public-private partnership is Business Iceland’s responsibility to market Iceland abroad and promote economic growth in Iceland.
Akansha Dimri, co-founder and CEO of TFN, met with some of the most exciting Nordic startups contributing to the economy, creating jobs, and expanding globally while attending the program. Her other stops include the Ok, Bye Climate Summit in Reykjavk’s main concert hall and the startup hub, Gróska, the biggest in the city. Check out the companies roaring in the technology sector of the country.
- Alvotech
A small network of biosimilar specialists works at Alvotech’s Reykjavik headquarters, which covers 13,278 square meters. Alvotech decided to engage international collaboration between scientists, engineers, architects, and Icelandic artists to create the best possible scientific environment and work environment.
The Alvotech range includes the Alvotech & CCHT Biopharmaceutical joint venture based in China. In an exclusive partnership, Yangtze River Pharmaceutical Group has partnered with Changchun High & New Technology Industries (Group) Inc. to commercialize eight biosimilar medicines across China.
As part of the initial pipeline are biosimilar candidates slated for treatment in the fields of ophthalmology, oncology, autoimmunity, and severe inflammatory conditions.
The Yangtze River Pharmaceutical Group is one of the largest pharmaceutical companies in China, and we’re pleased to announce this new partnership as we continue to progress. Through this commercial relationship, Yangtze River Pharmaceutical can access the strong brand reputation and market experience of Alvotech and Alvotech & CCHT Biopharmaceutical Co., Ltd.
Creating an integrated biosimilars powerhouse in the region will improve access to high-quality biosimilar medicines,” says Robert Wessman, Founder, and Chairman of Alvotech.
As part of this contract, Alvotech and Alvotech & CCHT Biopharmaceutical Co., Ltd, jointly established in 2005, are responsible for developing, registering, and supplying their biosimilars in China, while Yangtze River Pharmaceutical will commercialize them exclusively. In addition, Alvotech recently announced partnerships with Teva Pharmaceuticals for North America, Stada for key European markets, and several others.
CCHT Biopharmaceutical Co., Ltd. and Alvotech is collaborating on developing biosimilar medicines. The company’s vision includes designing, manufacturing, and introducing high-quality biosimilars. To deliver biosimilars with extensive clinical needs to the broad China market, we want to use our brand advantage and market experience fully.
As a result, we are committed to caring for all and dedicated to improving for the better. Alvotech and Alvotech & CCHT Biopharmaceutical Co., Ltd. have a leading innovative capability and technology. Therefore, we can be confident that the biosimilar medicines we give Chinese patients will be safe and effective. Founder and Chairman of Yangtze River Pharmaceutical Group, Xu Jingren, believes this partnership will be constructive, complementary, and benefit all participants.
CCHT Biopharmaceutical Co., Ltd, a partnership between Alvotech and CCHT, will manufacture these biosimilar candidates in Changchun, China1. In addition to single-use disposable bioreactors, the manufacturing facility will have the latest best-in-class technology. It is scheduled to open in 2021.
A global standard antibody drug production and manufacturing platform will be built with Changchun Alvotech and CCHT Biopharmaceutical Co., Ltd. Furthermore, to improve the accessibility and availability of biosimilar drugs, both parties could utilize their advantages in exploring China’s biosimilar market and improve people’s lives through their partnership with Yangtze River Pharmaceutical Group. In the future, CCHT and Alvotech & CCHT Biopharmaceutical will discuss cooperation with YZR at higher levels,” says Ma Ji.

Company overview
|
Legal Name |
Alvotech |
|
Industries |
Biotechnology, Life Science |
|
Founder(s) |
Robert Wessman |
|
Founded Date |
2013 |
|
Total Funding Amount |
$450,000,000 |
|
Investors |
Number of investors: 6 |
- Oculis
Innovate breakthrough therapies for addressing important unmet medical needs in the eye, improve eye care and save sight at Oculis, a global biopharmaceutical company. Oculis’s highly differentiated pipeline includes treatments for topical retina, biologics, and disease-modifying products. Oculus is poised to give patients worldwide life-changing therapies due to its presence in key international markets. Its headquarters are in Lausanne, Switzerland, and it has operations in Europe, the United States, and China under the leadership of an experienced management team.
In a licensing agreement issued today, Oculis S.A., a global ophthalmology company specializing in developing eye-care technologies, and Accure Therapeutics, a private translational neuroscience research company, have exclusive rights to develop and commercialize ACT-01, potentially disease-modifying treatment for protecting and preventing optic nerve and retina damage.
ACT-01, which is being renamed OCS-05, demonstrated positive results with neuroprotective properties in animal models of neuroinflammation and neurodegeneration. IGF-1 and BDNF are trophic factor pathways activated by their mechanism of action. With OCS-05, chronic vision loss is prevented in ophthalmology conditions where the nerve axons are protected, like acute optic neuritis and glaucoma.
Phase 2a (ACUITY) is currently being conducted in healthy volunteers after a phase 1 safety, and the PK study produced positive preclinical results.
In ACUITY, patients with acute optic neuritis are compared with OCS-05 using two arms, a double-blind, placebo-controlled study. Aside from safety measures, secondary outcomes will include optic nerve anatomical and visual function assessments. As part of the Public University Hospital Group in Paris (APHP), the study is taking place at La Pitié-Salpétrière hospital in Paris. More than one-third of the participants are currently enrolled in the study.
Among other regulatory agencies, Oculis is in the process of expanding its ophthalmology program.
Riad Sherif, MD, CEO of Oculis, proclaimed, “This partnership combines Accure’s unique neuroprotective approach with Oculis’ expertise in ophthalmology, helping transform the treatment of neurodegenerative diseases.”. It’s a perfect match for Oculis’ goal to develop ocular unmet medical needs treatments through innovation and differentiation. Glaucoma remains the leading cause of irreversible blindness globally, even with IOP-lowering treatments. It could be our first neuroprotective for optic neuropathies in the form of OCS-05.”
It is now the right time to partner with two like-minded companies committed to patients and driven by a passion for neuroscience, said Laurent Nguyen, M.D., Co-Founder and Chief Executive Officer of Accure Therapeutics. In addition, culis has a potential ability to leverage its expertise in ophthalmology to treat a wide range of neurodegenerative diseases.”
Based on reports of wild-type experimental animal models of acute optic neuritis induced by acute inflammatory demyelinating of the optic nerve (an acute inflammatory demyelinating disorder of the optic nerve) and high-pressure glaucoma (a condition causing permanent blindness after exposure to high eye pressure), OCS-05 reduces the destruction of the optic nerve and retina. In addition, in an animal model for multiple sclerosis, autoimmune encephalomyelitis (inflammation of the brain and spinal cord caused by the body’s immune system) improves paralysis.
Company overview
|
Legal Name |
Oculis |
|
Industries |
Biotechnology, Health Care, Pharmaceutical |
|
Founder(s) |
Einar Stefansson, Thorsteinn Loftsson |
|
Founded Date |
2003 |
|
Total Funding Amount |
$94,355,910 |
|
Investors |
Number of investors: 14 |
- Controlant
In industries across the globe, such as pharmaceutical and food, tracking cold chain temperatures is an essential part of ensuring safety and quality control. Wessex Power uses Controlant, an innovative and industry-trusted system, to help facilitate visibility throughout their supply chains. Here’s why Controlant has successfully monitored cold chains for so many different organizations.
You can track your shipments from start to finish with Controlant’s cold chain tracking system. A cold chain data logger tracks these metrics and connects directly to the Controlant platform. All data is securely stored in the cloud so that they can access it from anywhere in the world.
The software lets you monitor real-time temperature data and analyze your shipments’ journey once they have been delivered. During the cold chain, organizations can make modifications to loads if necessary. Take a look at Controlant’s advanced features and learn more.
Controlant is already providing organizations with increased visibility across their entire supply chain due to its cold chain temperature monitoring capabilities. Furthermore, Controlant has enabled companies to comply with government regulations and maintain quality controls throughout the cold chain. Controlant helps facilities comply with temperature regulations and ensure that their products are distributed safely.
Our team at Wessex Power used Controlant to improve real-time temperature visibility through the entire cold chain for an organization that distributes COVID-19 samples. To guarantee that the models reached the laboratories for testing at safe temperatures, they were stored at these temperatures before they got to the laboratories. In the case of ineffective temperature control, samples would be deemed inconclusive, wasting time and resources. They can prevent this by using Controlant.
Pharmaceutical company Teva, which develops and distributes medicines, also uses the Controlant cold chain monitoring system. One of the most appealing aspects of Controlant was real-time temperature monitoring. Whenever safe temperatures are breached, they want a quick solution to minimize problems rapidly. They also desired a product that could predict a potential issue before it could jeopardize product quality or the company.
Cold chain data loggers and the Controlant platform have already prevented 30 shipments from being delayed by Teva. Additionally, they stopped one of the most expensive and critically needed vaccine shipments from entering a room where they could not maintain the temperature. This prevented product wastage on a massive scale by alerting the company to a problem before it was too late. Through cold chain monitoring, Teva can eliminate as many potential temperature issues as possible.

Company overview
|
Legal Name |
Controlant |
|
Industries |
Developer APIs, Internet, Mobile, Software, Supply Chain Management, Internet of Things |
|
Founder(s) |
Gisli Herjolfsson, Stefan Karlsson, Erlingur Brynjulfsson, Trausti Thormundsson |
|
Founded Date |
2007 |
|
Total Funding Amount |
$54,722,277 |
|
Investors |
Number of investors: 13 |
- CCP Games
This year’s chaos has come to a close with CCP Games’ EVE Online: Phoenix unveiling of the fourth and final quadrant. When the galaxy rises from the ashes, the players will start the healing and rebuilding process again as the galaxy begins anew in the face of random attacks, war, and eventually, survival mode. Once you update, all the new options will appear in the game. Below you can find the latest info from the developers and a trailer showing it off. In addition, there is more information from the developers. So, where will you go from here?
Bergur Finnbogason, EVE Online’s Creative Director, described the success of the Triglavian Collective this year as wreaking havoc upon New Eden and destroying both star systems and the lives of its citizens. We are taking Capsuleers beyond the invasion to ask: “How do you shape your future?” By shifting to Quadrants in 2020, we’ve been able to dream bigger and create more meaningful impactful content.
A Triglavian invasion has profoundly affected the lives of the citizens of New Eden in Quadrant 4: Phoenix, a universe ravaged by war. Since the Triglavian Collective occupied many star systems during their invasion, New Eden has irrevocably changed. Their cause has been taken up by pilots who have taken up arms against them, and some have been forced to relocate to other systems. In addition, specific systems will be permanently inaccessible through conventional means due to interstellar travel disruptions across the cluster.
In the Phoenix Quadrant, the enormous Supercarrier class ships in EVE will experience a important update, including the addition of a new clone vat bay feature that will let pilots spawn new ships directly from a Supercarrier’s bay if they are destroyed in battle, allowing players to rejoin the action. In addition, the Crimson Harvest and Yoiul Festival, which will return to commemorate the end of the year and provide players with new personalization options and content, will take place as the Quadrant develops.
This will incentivize pilots to undock and participate as new daily challenges are available and developed. The wildly successful Abyssal Proving Grounds program will continue with new game modes, ship classes, and leaderboards. In addition, pilots will be able to explore the chaotic and lawless Abyssal Deadspace and engage in furious PvP battles to prove their worth and prevail.
Company overview
|
Legal Name |
CCP Games |
|
Industries |
MMO Games, Online Games, Video Games |
|
Founder(s) |
Reynir Hardarson, Sigurdur Arnljotsson |
|
Founded Date |
2007 |
|
Total Funding Amount |
$66,300,000 |
|
Investors |
Number of investors: 3 |
- Avo
Y Combinator, widely regarded as the world’s leading accelerator, has accepted Avo, Iceland’s SaaS startup preventing human error in analytics implementation. Y Combinator funds the company $150,000 as part of the program. An earlier seed round of $1.2 million was raised by local investors Brunner and Investa for Avo (then called Viska Learning).
Some of its founding team members worked for QuizUp, which attracted over $40m in venture capital from investors including Sequoia Capital and amassed 100m users before being acquired by Glu Mobile.
According to Stefania Olafsdottir, co-founder and CEO of Avo, “Every company is already using or soon will be using analytics mechanisms such as Amplification, Segment, Looker, Google Analytics or even their custom pipelines.” But humans don’t do well with analytics implementation.
According to Stefania, this problem also occurred at QuizUp, their previous workplace. “To help alleviate that problem, we created our analytics validation tool in-house.”
They reencountered this problem after starting a second company. It seemed absurd that they would need to create internal tools again, so they looked at what colleagues and friends were doing.
The surprising number that has this problem and hates it, or have fixed it by creating tools like what QuizUp did,” Stefania says. We knew we’d hit a home run then.
It would seem that Y Combinator partners also believe they are onto something.
Airbnb’s former growth lead and partner with YC, Gustaf Alströmer, said in a statement: “I always wanted something like this at Airbnb.”
In our experience, we had many problems such as accidentally removing analytics implementation when we refactored or launched new flows, misspelled program names and incorrect funnels that took until release version 16 to fix, and poor knowledge of where to find the meaning of programs among other teams. Avo can solve all this!”
Although Avo is the first Icelandic startup to join Y Combinator, it is not the first Icelandic company. The Helgason brothers, Ari and Ingvar, participated at YC with their companies in 2012 and 2017, respectively.

Company overview
|
Legal Name |
Avo |
|
Industries |
software |
|
Founder(s) |
Dekel Valtzer |
|
Founded Date |
March 1, 2017 |
|
Total Funding Amount |
$1,450,000 |
|
Investors |
NA |
- Kerecis
In addition to cellular therapy, tissue regeneration, and tissue protection, Kerecis develops products made from fish skin and fatty acids. Integral fish skin protects and regenerates tissues in the body. In addition, infections caused by bacteria and viruses are prevented by Kerecis sprayable topical and oral formulations.
Currently, Kerecis is the only company globally authorized to manufacture medical devices containing intact fish skin.
A report from SmartTRAK Business Intelligence claims it is the nation’s fastest-growing skin and dermal substitutes company.
The Sustainable Development Goals are a priority for Kerecis. In the town of Isafjordur, near the Arctic Circle, Kerecis processes 100% renewable energy to produce its fish skin products from wild and sustainable Icelandic fish stock.
A team from our production department traveled to the Westfjords of Iceland last week to explain and document a wound care product developed by Kerecis. In addition to pristine landscapes, geothermal activity, and wildlife, there were also stunning views along the way.
Fishing makes up 13% of Kerecis’ economy, located within the town of safjörour. One of the bi-products of fishing is the fish’s skin, which is used to make other products. It is rarely wasted. Kerecis discovered that the skin from cod fish fastens and supports wound healing in our bodies and that this fish skin has some remarkable properties. Kerecis has proven resourceful and innovative by taking something that would typically be discarded and turning it into a lifesaving product. A product like this can save limbs in many cases.
Any other country cannot match Iceland’s natural beauty. A collision of the North American and Eurasian tectonic plates creates a stark contrast between the high peaks, deep ravines, white snow, black volcanic ash, blue waters, and green moss covering the entire island. To top it off, the steam rising from the bubbling hot springs creates a weird sense of wonder.
As a result of the FDA’s approval, Kerecis Omega3 SurgiBindTM is now available for sale in the United States. As a result, a fish-skin graft implantable for plastic and reconstructive surgery is now available in the United States.
Soft tissue reinforcement, or reinforcement in plastic or reconstructive surgery, is achieved by implanting the product in weak areas.
As the first physician to use SurgiBind commercially outside of a study, Dr. Hector Crespo Soto of Atrium Health’s Sanger Heart and Vascular Institute has used it in his practice. In a ray amputation surgery, I used the product with great success. Incisions stayed closed after surgery and healed quickly. With Kerecis, surgical procedures are enhanced, a bacterial barrier is created, and the body’s healing mechanism is reinforced.”
Practitioners can better manage risks and achieve better outcomes with Kerecis Omega3 SurgiBind. Fish-skin technology accelerates full tissue remodeling by providing closed-cell ingrowth and incorporation, facilitating neovascularization, and speeding wound closure. Incisions in the abdomen, surgical flaps, and hip replacements may benefit from the reinforcement of soft tissues.
In addition to treating severe wounds and preventing amputations, this new product reinforces Kerecis’ entry into the surgical market.
A graft of Kerecis Omega3 transforms damaged human tissue into living tissue by recruiting the body’s cells. Kerecis fish skin is made only gently and retains its resemblance to human tissue due to the lack of disease-transfer risk between cold-water fish and humans. In addition to preserving the skin’s original three-dimensional structure, gentle processing keeps its inherent natural strength, complexity, and molecules. In clinical trials, Kerecis products have been found to heal wounds faster than their competitors.
Kerecis Omega3 SurgiBind targets the surgical market in their first implantable medical device. Kerecis also offers SurgiBind, SurgiClose MicroTM, and GraftGuideTM for surgical applications, in addition to SurgiBind.
Kerecis Omega3 technology can be applied beyond skin grafts for severe wounds with the introduction of SurgiBind, further strengthening Kerecis’ entry into the surgical market.
Company overview
|
Legal Name |
Kerecis |
|
Industries |
Health Care, Manufacturing, Medical, Medical Device |
|
Founder(s) |
Baldur Tumi Baldursson (MD)Hilmar Kjartansson (MD), J. Ernest Kenney, Gudmundur Fertram Sigurjonsson |
|
Founded Date |
2009 |
|
Total Funding Amount |
$48,611,641 |
|
Investors |
Number of investors: 7 |
- Authenteq
It has taken just two weeks for Berlin-based Authenteq Ltd. to close a $5 million funding round.
$7 million has been invested in Authenteq since 2015 from Draper Associates and Capital300 GmbH in the company’s Series A round.
In highly sensitive industries like finance that need to adhere to anti-money laundering regulations, Authenteq provides a blockchain-based distributed ledger solution for identity verification.
According to Tim Draper from Draper Associates, who invest in Twitter, Uber, Tesla, Coinbase, and Twitch Interactive, Authenteq represents a breakthrough in true and pure identity, which could eliminate many security concerns in the market today.
Also, the startup offers Trollteq, a service that deals with the problem of trolls on social media. Community managers can use this blockchain system to manage identity and prevent hostile users from making additional accounts, known as “sock puppets,” to avoid punishments and bans.
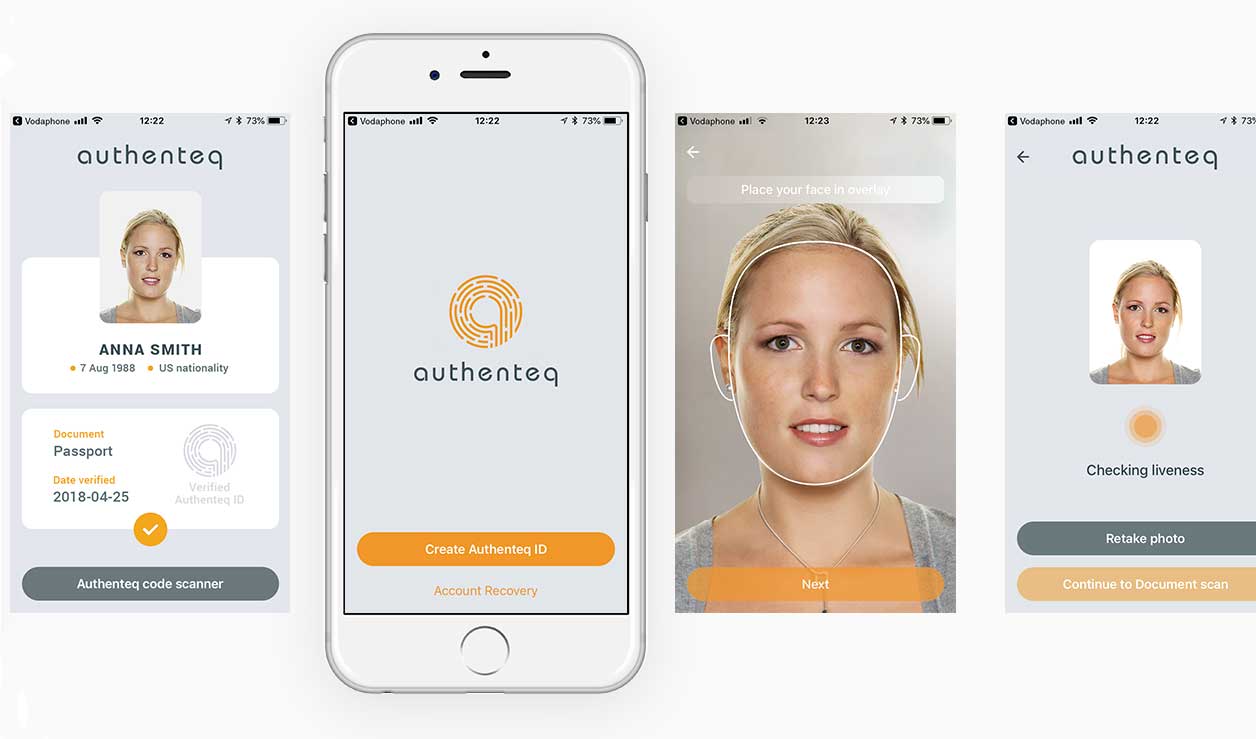
Authenteq uses facial recognition technology to verify the identity of customers by confirming their photos and identity documents, such as passports and other government-issued IDs. An “eID” is created on the phone by encrypting and duplicating the person’s image, along with certain important information.
To verify that a user is who they claim to be, a company can use the mobile phone as identification, comparing it securely against information on the blockchain.
A McKinsey and Co. report estimates that the global mobile verification market will reach $10 billion within four to five months and double by that time. Founder Peter Lasinger, who heads Capital300, noted that KYC and identity verification are growing in importance.
With offices in Berlin and Reykjavik, Authenteq currently employs 22 people. As a result of this new funding, the startup will be able to expand and hire for new positions in the identity management industry as new opportunities arise.
Kari Thor Runarsson, the chief executive of Authenteq, said they blessed them to have their offices in two of Europe’s hottest cities. In other words, it hasn’t been that difficult to convince talent to move to Berlin or Reykjavik from all over Europe and the United States.”
Over 150,000 electronic identification cards have been issued by the company’s system, more than Iceland’s national ID card program. In addition, Authenteq can accept more than 3,500 documents for identity verification, including photo IDs issued by most governments. Connected to more than 10,000 data sources worldwide and operating in more than 190 countries, the system can corroborate identities across the globe.
Draper Associates and capital300 led a $5 million funding round for Reykjavik-based Authenteq. This current round of capital is just “the first close in their Series A round,” the company said, adding that it is currently negotiating with investors for a second close.
According to Authenteq, its product uses AI to verify an individual’s identity in under 60 seconds. First, Authenteq’s mobile app reads the NFC data from an ID and compares it to a selfie taken by a user. After the app creates an “eID,” it encrypts and stores it on a private blockchain.
As part of the Trollteq launch, Authenteq also developed a platform for providing user authentication for online forums with the authentication platform.
We intend to use the funding to hire more employees. We currently employ 22 people in Berlin and Reykjavik.
Company overview
|
Legal Name |
Authenteq |
|
Industries |
Fraud Detection, Blockchain, Compliance, Identity Management, Privacy, Information Technology |
|
Founder(s) |
Adam H. Martin, Kari Thor Runarsson, Runar Karlsson |
|
Founded Date |
2015 |
|
Total Funding Amount |
$7,398,103 |
|
Investors |
Number of investors: 8 |
- Lucinity
Founded in 2015, Pleo delivers innovative spending solutions to forward-thinking businesses through a simplified approach to spending management. Pleo has exponentially acquired new customers, serving various industries and companies worldwide. Customers were searching for a solution that could provide a dynamic, modern approach to transaction monitoring without impacting their AML monitoring process’s security or integrity.
The AI-based Anti-Money Laundering solution from Lunity supports human insights and increases compliance professionals’ efficiency and productivity. Pleo’s highly scalable products and user-friendly API made it the perfect choice for supporting its rapid growth.
As part of its AML compliance strategy, Pleo will use Lucinity’s comprehensive AML software solution that includes transaction monitoring, case management, actor intelligence, and suspicious activity report management. In addition, Pleo’s compliance team will be able to gain actionable insights through Lucinity’s simple and stable technology because both products are simple and stable.
Through its open design, Lucinity’s anti-money laundering software can also integrate with Denmark’s Financial Intelligence Unit (FIU), so Pleo can manage tax returns more efficiently, reducing the review and grading process for tax returns from four hours to minutes. The method also allows Pleo to grade its customers’ tax returns directly.
Pleo’s Chief Compliance Officer and Money Laundering Reporting Officer, Charlotte Lowry, commented: “Lucinity’s AI-powered anti-money laundering software allows for smarter and more actionable anti-money laundering (AML) oversight, which we have been searching for as we pursue a solid foundation of AML compliance risks.
Collaboration has been driven by the proactive approach and superior interface of Lucinity. We are looking forward to growing with them together. “
Gu*mundur Kristjánsson, Founder and CEO of Lucinity, said, “We believe that by transforming AML compliance and coming up with a smarter solution, we can achieve important change for society.” In partnership with Pleo, we look forward to providing customers with progressive and intelligent solutions.”
French fintech Formance raises €2.9m to help companies create and track cash flows. Founded in 2021, the startup offers a low-code solution for creating, tracking, and implementing real-time payment flows that can be used by companies, marketplaces, platforms, and fintech. Hoxton Ventures leads the round. According to Formance, it is currently piloting its solution with an undisclosed fintech.
Pleo chooses Lucinity
Danish fintech Pleo chooses Lucinity as its central hub for its compliance risks. The AML solution uses AI systems to support compliance processes. According to the two companies, Pleo will deploy Lucinity’s full AML compliance software, including transaction monitoring, case management, actor intelligence, and SAR Manager, to process suspicious activity reports (SARs).
Visa launches new DPS service with DKB.
The issuer processing service “Visa DPS” is one of the largest payment processors for Visa transactions worldwide. For example, 50 percent of all Visa debit transactions in the USA are processed through it. The DKB is now the first bank in Europe to use the processing service Visa DPS. As Visa emphasizes in the press release, partner banks can easily integrate Visa DPS into their existing infrastructure via APIs. The system supports digital cards, including instant digital card issuance and card and account details management. Consumers also benefit from this, as the report states. In addition, you benefit from features such as digital card management, setting limits, an overview of expenses, card blocking, and choosing a PIN.
Founded in 2015, Pleo provides intelligent spending solutions for forward-thinking businesses through a streamlined approach to spend management. Pleo has added new customers exponentially, serving various industries and companies worldwide. They were looking for a reliable solution that offered a dynamic and modern approach to transaction monitoring that could scale quickly without compromising the security and integrity of their anti-money laundering (AML) processes.
Lucinity’s AML solution uses artificial intelligence (AI) to human power insights, increasing the efficiency and productivity of compliance professionals. The highly scalable products combined with the integration-friendly application programming interface (API) were the ideal solution to support Pleo’s rapid expansion.
Pleo will deploy Lucinity’s complete AML compliance software, including transaction monitoring, case management, actor intelligence, and SAR Manager, to process suspicious activity reports (SARs). With the simplicity and stability of Pleo and Lucinity’s products, Lucinity will empower Pleo’s compliance team to receive actionable information through visually appealing and trusted technology.
Due to its open design, Lucinity’s AML software can also be integrated with the Danish Financial Intelligence Unit (FIU), enabling Pleo to manage suspicious activity reports more efficiently, reducing the review and filing process from four hours to minutes.
Charlotte Lowry, Money Laundering Reporting Officer (MLRO) & Compliance Director, Pleo, commented, “Lucinity’s AI-powered AML software enables a smarter and more actionable approach to AML monitoring – something we’ve been looking for since we Scale with a robust AML compliance risk base. In addition, the company’s collaborative approach, coupled with an excellent interface, was a key reason for working together. We are planning to make a close partnership with them as we continue to grow.
“We believe that by transforming AML compliance and applying a smarter approach, we can have a important positive impact on society,” added Guðmundur Kristjánsson, Founder and CEO of Lucinity. “Pleo shares our vision of progressive and intelligent solutions for forward-thinking customers, and we look forward to working with them.”
Investors probably don’t want to save on Celsius.
This is not good news for Nuri customers who have chosen the income account. Because according to a report by the WJS, to which other media refer, the fintech Celsius, which has stumbled, is not on the verge of being rescued by its investors. Last year, Celsius raised $750 million in a Series B round. If the report is correct, none of the investors involved is currently willing to add more money. The unanimous opinion should be that the risk was significantly greater than the investors had estimated.
McKinsey sees German fintech left behind.
The German fintech landscape is falling behind in a European comparison. This results from a new McKinsey study, which is available to the Handelsblatt. Although the fintech from Germany is currently among the most valuable financial service providers, they lag behind competitors from other European countries. Therefore, it is high time to act. The study authors are quoted as saying. The authors of the study see four factors for the poor performance of German fintech: the startup rate, scaling, financing, and regulation.
Ant is taking another step toward the stock market.
Last week, Bloomberg reported that Ant Financial, the Chinese fintech behind Alipay, is making another bid toward an IPO. This had been banned at short notice by the Chinese authorities. Now Reuters follows up: According to this, the Chinese central bank has accepted an application from Ant to set up a financial holding company. It is expected that the authority will agree to this.
Company overview
|
Legal Name |
Lucinity |
|
Industries |
Artificial Intelligence, Software |
|
Founder(s) |
Gudmundur Kristjansson |
|
Founded Date |
2018 |
|
Total Funding Amount |
$8,100,000 |
|
Investors |
Number of investors: 2 |
- GRID
New Enterprise Associates (NEA), one of the world’s largest and most active venture capital firms, led a Series A round of approximately €10.1 million for Icelandic startup GRID today, including BlueYard Capital, Slack Fund, Acequia Capital, and other strategic partners. As a result of this financing, the company will be able to bring its product to market and accelerate the development of the product.
Hjalmar Gislason and fellow software veterans founded GRID in the fall of 2018. With an extensive background in data services and analytics, the team recognized that spreadsheets are essential to any data transformation process. Data science notebooks have made communication and reporting of data and models easier and more powerful using Excel and Google Sheets, leveraging everyday people’s spreadsheet skills and assets.
According to Hjalmar Gislason, CEO, and founder of GRID, data reporting and modeling are only the beginning. We need to change how we think, report, and collaborate around data because data is ubiquitous in organizations today. By understanding and leveraging people’s existing workflows, rather than trying to introduce something completely new, we can ensure that they will continue to work the way they already do. NEA is the best possible partner for us in achieving this vision, and we’re thrilled to have them join us.”
The NEA General Partner, Forest Baskett, has long recognized that Excel is the world’s most popular coding language. As a company that is not only able to augment spreadsheets but also to build a stand-alone business that is financially viable, Grid is entering the market as a company that can enhance spreadsheets. The NEA has seen first-hand how B2B companies have grown through product-led growth. The Grid team has extraordinary potential, and we look forward to helping them propel the go-to-market and reinvent spreadsheet use.”
Over the past few months, Grid has built a community of enthusiastic users and improved its user experience and functionality.
Company overview
|
Legal Name |
GRID |
|
Industries |
Productivity Tools, SaaS, Software |
|
Founder(s) |
Hjalmar Gislason, Thorsteinn Yngvi Gudmundsson |
|
Founded Date |
2018 |
|
Total Funding Amount |
$16,540,347 |
|
Investors |
Number of investors: 14 |
- TripCreator
Each year, the Web Award Marketing Association awards prizes to outstanding websites in several categories. TripCreator, a tailor-made itinerary generator, received the title of Best Travel/Tourism Website.
The WebAwards are the premier annual competition for web developers and marketers.
The 2015 edition took place before a jury of experts represented by 96 companies.
On a scale of 0 to 10, websites are judged on several criteria: aesthetics, innovation, content, technology, interactivity, writing, and ease of use.
Created in 2013 by Hilmar Halldórsson, TripCreator is an Icelandic platform generating tailor-made itineraries.
It scored 68.5 points out of 70, equating to a score of 9.8 and the award for Best Travel/Tourism Website 2015.
“The information is numerous and well presented on the site. Every detail has been thought out so the traveler can organize a simple or complex trip and benefit from multiple recommendations for activities and places to discover.
It’s a beautiful and highly interactive site that will speak to travelers, its primary audience. Great job!” commented the judges.

Company overview
|
Legal Name |
TripCreator |
|
Industries |
Travel Agencies, Travel Services, OTAs, Airlines, and Bloggers/Indie Media |
|
Founder(s) |
Hilmar Halldorsson |
|
Founded Date |
Jan 1, 2013 |
|
Total Funding Amount |
$8,020,053 |
|
Investors |
NA |
edited and proofread by nikita sharma




