The WHO may issue a warning against cough syrups for children, according to a new report.

According to a source acquainted with the situation, the World Health Organization (WHO) is looking into whether there is any connection between the manufacturers of the alloyed cough syrup that it has connected to the deaths of more than 300 children in three nations.
The individual said that the WHO is interested in learning more regarding the precise raw components employed by six manufacturers in India and Indonesia to create the medications related to the most recent fatalities, as well as whether or if the organisations bought them from several of the identical vendors. The individual mentioned “lesser scenarios” of toxins in the area.
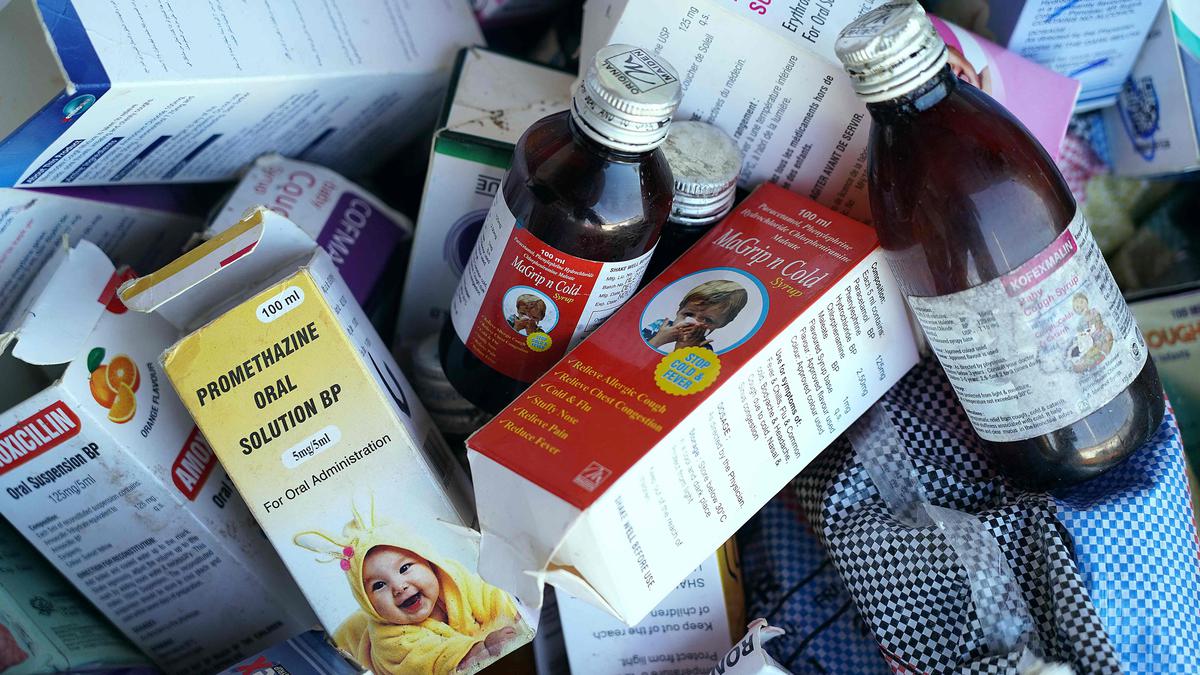
The WHO is also debating whether to advise families globally to reconsider using cough syrups for children generally in light of current research into the safety of certain of these medications. The individual claims that WHO specialists are examining the data to see if children’s medical needs require comparable information.
Injuries to children with acute renal failure first occurred in the Gambia in July 2022, and later cases spread to Indonesia and Uzbekistan. The deaths, according to the WHO, were brought on by young people taking unsafe cough medicines for common ailments that included either diethylene glycol or ethylene glycol, two known toxins. The WHO has so far connected six medicine producers in India and Indonesia to the bathos. These directors either declined to acknowledge the investigation or denied utilising risky items that contributed to any losses.
WHO prophet Margaret Harris noted, “This is of the biggest concern for us, to see no future child losses from commodities that are so preventable, without providing any new information regarding the details of the association’s plan.
The health organisation of the United Nations revealed on Monday that it has extended its investigation into ethylene and diethylene glycol contaminants in cough baths to four additional countries, including Cambodia, the Philippines, East Timor, and Senegal, where the same products may have been sold. It sparked swift investigations to weed out unreliable information and improve governance in other countries and the global pharmaceutical business.
Later on Tuesday, the WHO is expected to provide further insight on the cough sugar issue during a news conference. In October 2022 and earlier this month, the WHO issued clear warnings against cough baths produced by two Indian companies, Maiden Pharmaceuticals and Marion Biotech. Separately, the reports said that the use of these baths had been connected to fatalities in both the Gambia and Uzbekistan.
Both Marion and Maiden’s production facilities have been put on hold. Maiden is currently attempting to renew after the Indian government announced in December that its tests had found no problems with its products. Reuters has received continuous assurances from Maiden that it has not done anything impolite, most recently in December. Naresh Kumar Goyal, the managing director, stated on Tuesday that he had no comment about the WHO’s investigation into any connections between the businesses under review.
Maiden has constantly assured Reuters that it has done nothing indecorous, most recently in December.
On Tuesday, no one picked up at Marion’s office, and the company didn’t immediately return a dispatch asking for a comment. Due of its proximity to New Delhi, it warned the government of Uttar Pradesh earlier this month that the losses in Uzbekistan were being connected to it “to tarnish the image of India and the establishment.” Cough baths made by four Indonesian firms and sold locally were the subject of a warning from the WHO and Indonesia’s pharmaceutical enforcement agency in October.
When asked about the WHO’s probe into possible ties between the losses in the three nations on Tuesday, PT Yarindo Farmatama, PT Konimex, and PT AFI Farma did not directly comment. Hermansyah Hutagalung, the lawyer for PT Universal Pharmaceutical Diligence, claims that the company has removed all of the requested cough medicines from its inventory. Hutagalung suggested going after the suppliers since they were the true lawbreakers.
That fabricates raw component attestation up to pharmaceutical companies in order to manufacture bogus raw materials. He made the claim without identifying any specific suppliers or offering evidence to back it up.
Diethylene glycol and ethylene glycol, which the WHO described as “ppoisonous composites used as artificial detergents and antifreeze agents that may be murderous indeed with little attention,” were set up to be present in the baths, according to the WHO. Their toxic consequences include death, renal damage, and the inability to pass urine.
The losses have drawn attention to possible sins in the worldwide regulation of routinely used medicines, including control of manufacturing installations and supply chains, particularly those producing goods for impoverished nations that warrant the means to cover the safety of medicinals.
The WHO establishes worldwide norms for the manufacture of drugs and aids nations in their examinations of any excrescences, but it lacks the power to charge or enforce violators directly. After a disagreement between the Gambia and Uzbekistan over cough saccharinity, the WHO calls for action to guard children from tainted specifics. Tuesday saw the World Health Organization (WHO) issue a critical call to action for nations to help, descry, and address cases of shy and fake medical particulars.
“Over the past four months, multiple instances of untoward cough baths for kids being defiled with inordinate amounts of diethylene glycol (DDEG) and ethylene glycol have been reported by nations” (EEG). Over 300 losses have been reported in three of these countries as a result of the cases, which come from at least seven other nations. Most are youthful children, frequently under five years old. These pollutants—poisonous composites used as antifreeze and artificial detergents, according to the global health ministry—can be dangerous in small doses and should never be present in specific amounts. 
WHO has released three global medical warnings addressing this frequency, grounded in public reporting. The outbreak in the Gambia was the focus of the medical product cautions that were released on October 5, 20, and November 6, 2022; Indonesia was the subject of the alert that was released on November 6, 2022; and Uzbekistan was the focus of the alert that was released on January 11, 2023.
Since these circumstances aren’t unique,
WHO urged all major players in the medical supply chain to act quickly and cooperatively.WHO called on controllers and governments to identify any unacceptable medical particulars that have been stressed in the foregoing WHO medical warnings as possible causes of deaths and sickness and remove them from rotation in their separate requests? The global health organisation also made sure that all medicinal particulars in each request were available from authorised or certified merchandisers and had been given the go-ahead for trade by the applicable authorities.
The WHO also emphasised the importance of conducting thorough testing after entering inventories and before using them in the production of final goods. They also recommended only buying pharmaceutical-grade excipients from reputable sources. Also, as part of its charge to foster effective collaboration in the prevention, discovery, and response to unacceptable and fraudulent medical goods in order to save lives, WHO will keep working with the Member State Medium on these problems.
edited and proofread by nikita sharma




