100 million doses of covishield vaccines have expired and the production has been stopped in December 2021: SII
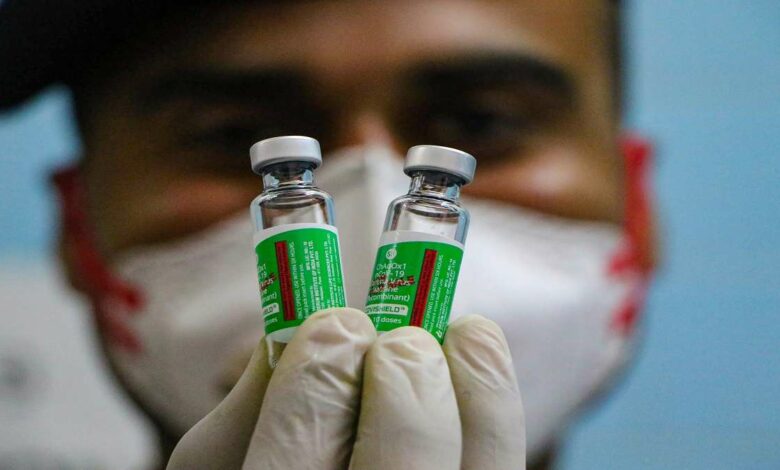
It has been confirmed that the Serum Institute of India (SII) stopped the production of the Covishield vaccine in December 2021. The firm’s CEO Adar Poonawalla said on Thursday that people are fed up with covid and vaccines.
One of the world’s most devastating health crises: covid-19 took a toll on the economy and led to the collapse of the health sector in several dominating nations of the world. The disease started as a small outbreak in China and has led to a long-term lockdown around the world.
This December would mark three years of the crisis. And it is common for people to be tired of the battling pandemic and its vaccines.
100 million doses of covishield vaccines have expired: Poonawalla
Poonawalla has opened up on the Developing Countries Vaccine Manufacturers’ Network (DCVMN) inaugurated in Pune on the 23rd general meeting that around 100 million doses of the covishield vaccine have gotten expired the total stock available at that time.

The covishield vaccine manufacturer has stated that developing new vaccines against the new strains of the Omicron strain will be time-consuming. But on a positive note, he has affirmed that the company has conducted trials for the covid vaccine Covovax as a booster dose and was seeking approval for the next 10 to 15 days.
At an event that was inaugurated by the Union Health Minister Poonawalla stated that the firm has already completed the trials as part of the DCGI requirement.
The global event brought 400 leaders, researchers, and scientists to a single platform from several global public health organizations, vaccine manufacturing industries, and research institutes.
When Poonawalla was further enquired about the production of the covishield booster dose and its update, he replied that the firm has stopped the production of covishield in December 2021. It was due to the reason that at least 100 million doses of covishield have expired, and the firm has stopped the production of covishield in December 2021.
He has remarked that there was lethargy in taking booster doses. People are tired of covid and taking vaccines. But, people can maybe take a flu shot every year in addition to the covid booster.
He further added that the experience does not seem much promising, and India has no culture of taking flu shots as it is dominant in Western countries.
The firm attempted to incorporate the idea into the H1N1 event, but it led to very little success.
Meanwhile, Poonawalla has even expressed hope about intranasal vaccines have the potential to reduce the transmission of the virus. He has stated that the firm will wait for the results of the Codagenix-Serum Institute of India’s single-dose intranasal Covid-19 vaccine before making any announcement.
The SII chief has observed that the clinical trials may take longer duration since the pandemic is virtually over, and the regulatory bodies may not work at a rapid pace like they performed when the covid-19 pandemic was at its peak.
Despite that, they believe that the Covovax booster dose vaccine will get approved in 10 to 15 days as they have finished the trials in India which was the requirement.
At the inaugural session, Dr. Mandaviya stated that around 70 percent of the country’s population has been vaccinated against covid-19. He has remarked that all health challenges can be faced and resolved when the power of the government, scientists, and industries is combined with national and international organizations.
The Board Chair DCVMN, Sai D Prasad has said a stable political environment is necessary to build a seamless global logistical and distribution access to vaccines. A strict, transparent, and strong regulatory system is essential, and value-based pricing is led by innovation-driven product development and multilateral associations.
He has further acknowledged the contributions of Cyrus Poonawalla and other members in the development of the covid-19 vaccines.
It has been brought to news that the Serum Institute of India is to supply 20 million doses of HPV vaccine to the Indian Government in 2023.
The vaccines would help to battle cervical cancer. The HPV facility was previously being used for the production of covid vaccines during the pandemic, and hence there was a delay in to fight against HPV.
A larger launch will be planned the next year, and the firms and the SII are matching up their sales to enhance the demands for India and export requirements.
edited and proofread by nikita sharma




