US CDC Report Links Indian Cough Syrups To Child Mortality in Gambia.
According to a joint investigation, many children's deaths in Gambia have been linked to the use of tainted cough syrups manufactured in India.

US CDC Report Links Indian Cough Syrups to Child Mortality in Gambia.
In recent years, the Gambia has been hit with a tragedy that has left parents and caregivers heartbroken – the death of children due to the consumption of Indian-made cough syrups.
According to a report by the US Centers for Disease Control and Prevention (CDC), these cough syrups contain dangerous levels of diethylene glycol (DEG), a hazardous substance that is employed in the production of industrial solvents and antifreeze. In this article, we’ll explore the issue in depth and shed light on the implications of this tragic event.
The CDC in the United States and the Gambian health department worked together to investigate the possible connection between the use of cough syrups manufactured in India and the deaths of 66 children in the Gambia.
According to a joint investigation, many children’s deaths in Gambia have been linked to the use of tainted cough syrups manufactured in India. “Our research indicates that a significant factor in this paediatric Acute Kidney Injury (AKI) cluster in The Gambia is the importation of medications containing Diethylene Glycol [DEG] or Ethylene Glycol [EG].” the CDC reported in a report released on Friday, as reported by news agency PTI.
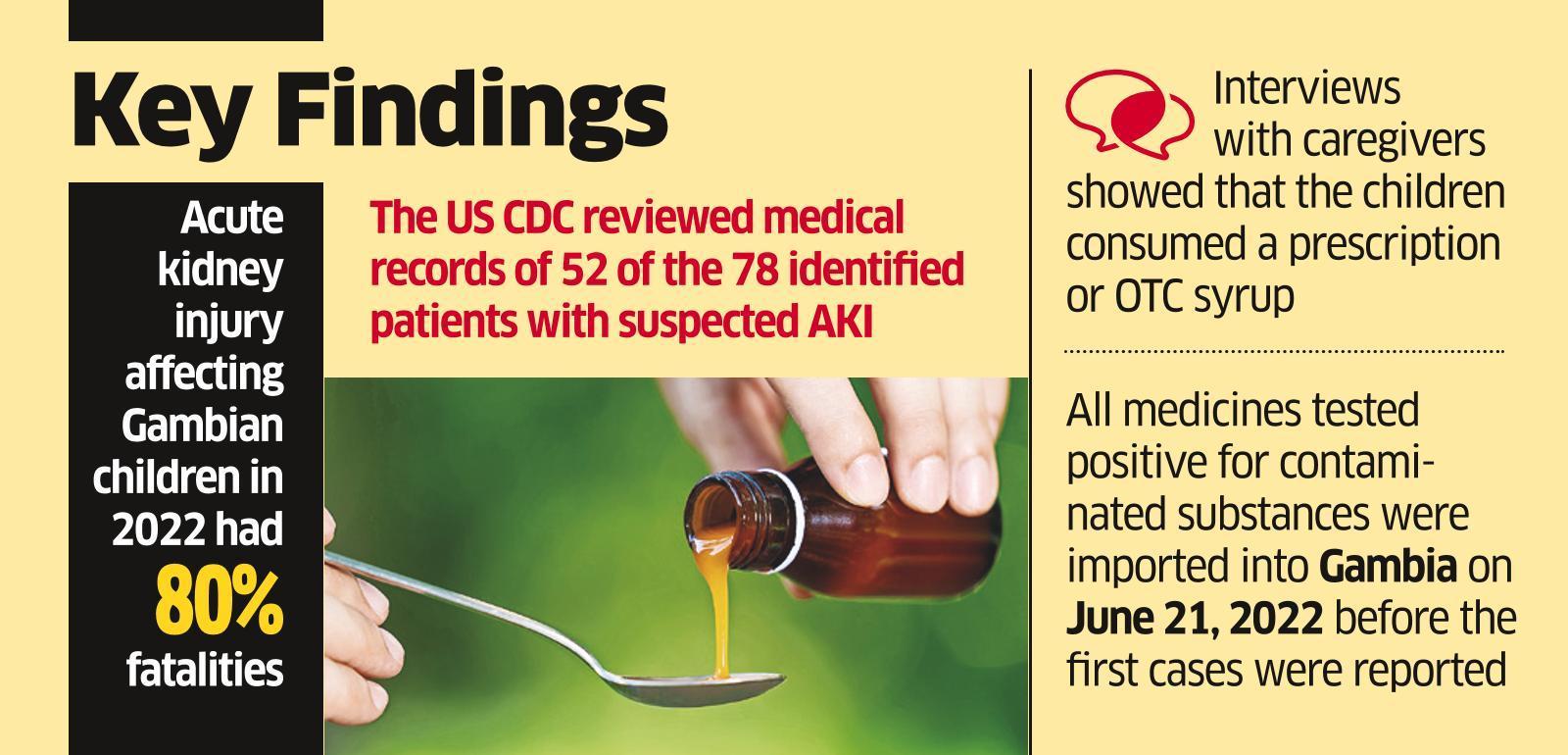
Alterations in mental status, headache, and gastrointestinal symptoms are all possible in patients with DEG poisoning. Still, acute kidney injury (AKI), manifested by oliguria (low urine output) or anuria, and progressing over 1-3 days to renal failure, is the most frequent presentation (indicated by elevated serum creatinine and blood urea nitrogen).
In August of 2017, the Gambia’s Ministry of Health (MoH) reached out to the CDC for assistance in defining the disease (many cases of Acute Kidney Injury and mortality in infants), outlining the disease’s epidemiology, and pinpointing its possible causes and origins.
According to the paper, manufacturers were likely using cheaper DEG as a stand-in for more expensive pharmaceutical-grade solvents during previous DEG outbreaks. According to the paper, this is the first time DEG-contaminated pharmaceuticals were imported into a country rather than produced locally.
In addition, the FDA stated that there was no evidence to suggest that the tainted cough and paracetamol syrups that were responsible for the deaths of children in Gambia last year had made their way into the US medicine supply chain.
The research also suggested that regulatory standards for export medicines could be laxer than for domestic usage. “At the same time, low-resource countries may lack the human and financial resources necessary to monitor and evaluate imported medications,” it said.
In early January, the FDA announced that it was assisting the World Health Organization and international regulatory authorities in their probe into tainted cough syrups that had killed almost 300 children across Africa and Asia.
The World Health Organization (WHO) issued a warning in October 2022, asserting that four cough syrups given to Gambia by India’s Maiden Pharmaceuticals Ltd were of poor quality and responsible for the deaths of numerous children in that country.

The supply network for Maiden Pharma reaches many different nations across Africa, Asia, Eastern Europe, the Middle East, and Russia. The business started in 1990, and in that time, it built a solid production line and made a name for itself in R&D.
Maiden Pharma has an export licence for four medications: Promethazine Oral Solution BP, KOFEXMALIN Baby Cough Syrup, MaKOFF Baby Cough syrup, and MaGrip n Cold Syrup.
The government cleared Maiden Pharmaceuticals in October, saying the company’s paediatric cough syrups are of “standard quality,” based on examinations of samples seized from the company’s Haryana factory. The administration has requested the samples from the World Health Organization and the Gambian government but has not received a response.
On 2/3/2019, Union Minister of State for Health Bharati Pravin Pawar replied to Lok Sabha saying that the cough syrup samples were tested and found to be of acceptable quality.
Pawar said in an email response that tests for the presence of Diethylene Glycol (DEG) and Ethylene Glycol (EG) showed that both were absent from the samples.
Another Noida-based pharmaceutical company had three employees detained on March 3 after their cough syrup was linked to the deaths of 18 infants in Uzbekistan in 2017.
The Marion Biotech employees were arrested after an FIR was filed against them. The authorities claimed that the Central Drugs Standard Control Organization’s (CDSCO) drug inspector filed the FIR.
Atul Rawat, Tuhin Bhattacharya, and Mool Singh have all been named as suspects who were detained at Marion Biotech Pvt Ltd. Samples of Marion Biotech’s medications were examined by federal and Uttar Pradesh state narcotics authorities before the arrests.
According to the complaining drug inspector, 22 of the samples tested were “not of standard quality” (adulterated and bogus) after inspection. In December of last year, Marion Biotech, with headquarters in Noida’s Sector 67, came under scrutiny after the CDSCO initiated an investigation into the possible link between its cough medication Dok-1 and the deaths of 18 children in Uzbekistan.
What is diethylene glycol (DEG)?
DEG is a colourless, odourless, and sweet-tasting liquid often used as a solvent in producing plastics, resins, and lubricants. While DEG is not inherently toxic, it can be lethal when ingested in large quantities. This is because it is metabolized into a compound that damages the kidneys and central nervous system, leading to seizures, coma, and, ultimately, death.
How did DEG end up in cough syrups?
In the Gambia, Indian-made cough syrups are a popular remedy for children suffering from coughs and colds. These syrups are sold under various brand names and are marketed as safe and effective treatments. However, investigations have revealed that many of these syrups contain DEG, which is used as a cheap substitute for glycerin – a safe and widely used ingredient in cough syrup.
The Indian manufacturers of these cough syrups have been accused of knowingly using DEG in their products, even though it is banned in most countries. This has led to widespread outrage and calls for stricter regulations on producing and importing pharmaceuticals in developing countries.
What are the implications of this tragedy?
The death of children due to the consumption of Indian-made cough syrups is a tragic reminder of the dangers of unregulated pharmaceutical production. It highlights the need for stronger regulations and oversight to ensure that pharmaceutical companies are held accountable for the safety and efficacy of their products.
Moreover, this tragedy underscores the importance of public health education in developing countries. Many parents in the Gambia were unaware of the risks associated with these cough syrups and trusted that they were safe based on the manufacturer’s marketing claims. By providing accurate and accessible information on the risks and benefits of various treatments, public health officials can empower parents to make informed decisions about their children’s healthcare.
What can be done to prevent such tragedies?
In response to the deaths of children in the Gambia, the government has taken steps to ban the importation and sale of Indian-made cough syrups. This is a positive development, but more must be done to prevent similar tragedies from occurring.
Firstly, there is a need for stronger regulations and oversight of pharmaceutical products in developing countries. This can be achieved through increased funding for regulatory bodies and closer collaboration between governments and international organizations.
Secondly, there is a need for greater public health education on the risks and benefits of various treatments. This can be achieved through public awareness campaigns, school-based health education programs, and community outreach initiatives.
Lastly, there is a need for increased transparency and accountability in the pharmaceutical industry. This can be achieved through greater scrutiny of pharmaceutical manufacturers and the establishment of ethical guidelines for producing and distributing pharmaceuticals.
In conclusion, the death of children in the Gambia due to the consumption of Indian-made cough syrups is a tragic event that underscores the need for stronger regulations and oversight in the pharmaceutical industry. It is also a reminder of the importance of public health education and the need for greater transparency and accountability in the production and distribution of pharmaceuticals. By taking these steps, we can work towards a safer and healthier future for all.
Edited by Prakriti Arora




