The quixotic search for an Alzheimer’s “Magic bullet” is prolonged by yet another failure.
What is Alzheimer's disease, and why has a "magic bullet" treatment been so difficult to find?

Clinical studies for an experimental Alzheimer’s medicine designed to halt or prevent cognitive loss in patients at high risk of developing the illness early were unsuccessful. This led to dealing with a setback and hopes to discover a ground-breaking cure for the neurodegenerative condition. What is Alzheimer’s disease, and why has a “magic bullet” treatment been so challenging to find?

What is Alzheimer’s disease?
Alzheimer’s disease is a degenerative brain condition that gradually impairs people’s memory and cognitive function. According to the World Health Organization, it is the most prevalent type of dementia or loss of cognitive functioning — thinking, remembering, and reasoning — occurring in 60 to 70 percent of cases worldwide (WHO).
It typically starts with a modest memory loss, but as symptoms worsen, patients lose the capacity to carry out even basic tasks. It is progressive and permanent.
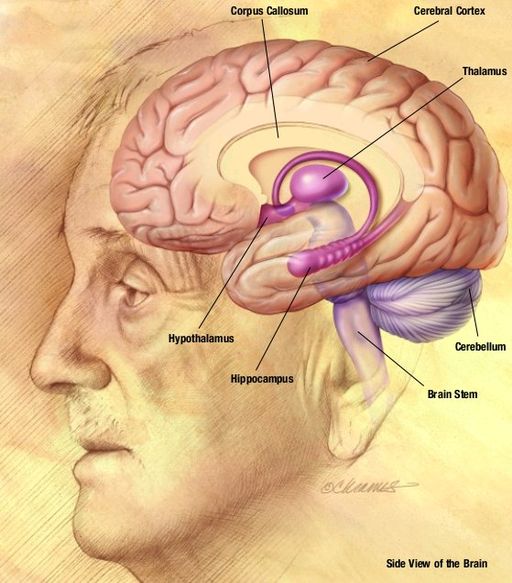
“Deposition of an aberrant protein called beta-amyloid in the brain is the disease’s pathology. Long before any symptoms appear, the disease has already begun. The pathology changes almost a decade sooner than the symptoms, according to Dr. Manjari Tripathi, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
When Dr. Alios Alzheimer examined the brain of a memory-loss patient after her death in 1906, he detected aberrant clumps (beta-amyloid plaque) and bundles of fibres (neurofibrillary tangles). The loss of connections between neurons necessary for the transmission of information inside the brain, combined with a plaque and tangle formation, are important indicators of the disease.

Recent trials that ended in failure
The ten-year project recruited participants with a particular genetic mutation that causes the early start of Alzheimer’s around the age of mid-40s and utilised crenezumab, a medication meant to inhibit beta-amyloid.
The United States National Institute on Aging, Arizona-based organisation Banner Alzheimer’s Foundation, and pharmaceutical company Roche all supported the experiment. It focused on the less common autosomal dominant form of Alzheimer’s disease (ADAD).
Crenezumab phase 3 trials for sporadic Alzheimer’s, which makes up over 90% of cases, were discontinued earlier in 2019 by Roche since they did not meet expectations.
Roche revealed this week that the outcomes of its most recent trial were also unsatisfactory and did not demonstrate “significant therapeutic advantages.” Researchers found that the neuropathological and clinical characteristics of sporadic AD and ADAD are comparable.
Why is this failure considered a critical setback?
For many years, a medication to both prevent and treat the memory-robbing condition has been elusive. According to the World Health Organization, dementia is currently the seventh most common cause of mortality worldwide and one of the main causes of disability and dependency in older people.
According to data reported in the journal of the Alzheimer’s Association, 121 distinct medicines were being investigated in 136 studies in 2020 to find a cure for AD (in the US). This medication development pipeline, which was taken from a survey of the clinical trials register maintained by the US FDA, sounds impressive—until it is put next to another figure.
Prior to this, researchers conducted a comparative study of the pipeline of AD treatments, examining 244 compounds in 413 clinical trials between 2002 and 2012, and discovered a startling 99.6 percent failure rate — compared to 81 percent for cancer. No medicine had advanced across the finishing line by the time an evaluation of the drug pipeline was released in 2020.
But in 2021, the USFDA authorised Biogen’s beta-amyloid-targeting medication aduhelm, making it the country’s first brand-new Alzheimer’s treatment in nearly 20 years. But after the fast-track approval, there was a heated argument among scientists about the trial’s results.
While that debate rages on, crenezumab’s failure has raised concerns about the bulk of Alzheimer’s studies’ treatment approach, which relies too much on neutralising beta-amyloid to combat the illness.
You cannot claim that eliminating beta-amyloid will solve all of your problems.
Such a complicated illness cannot be defeated with a single blow, according to Dr. Tripathi.
As of January 25, 2022, there were 143 medicines in 172 clinical studies for AD, according to the most recent drug pipeline evaluation published in May of this year.

What else is involved in the hunt for a treatment for Alzheimer’s?
Another strategy includes focusing on the tau protein, which misfolds and accumulates in brain cells as twisted fibres known as neurofibrillary tangles, rather than the harmful beta-amyloid plaque. However, medications that target each of these proteins separately—the “one drug, one target” model—have not yet made significant progress.
A pharmacological strategy that targets numerous enzymes and pathways that contribute to the development of the disease is another area of research that some scientists are concentrating on. This refers to multifunctional chemicals or multi-target-directed ligands (MTDL), which are thought to be a potential master key that might open numerous locks and seek to activate or modify multiple biological targets.
Another strategy is to halt the cognitive decline by altering modifiable lifestyle factors. This is the premise of the two-year FINGERS (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability) randomised clinical trial, which led to the expansion of the World-Wide FINGERS study. In the 1,260 participants aged 60 to 77, the FINGERS trial revealed a 40% improvement in cognitive performance and a 25% improvement in general cognition.
Genetics and age are unchangeable, irreversible elements in Alzheimer’s, according to Dr. Tripathi. Hypertension, diabetes, smoking, alcohol use, and a sedentary lifestyle are the strongest modifiable risk factors for the condition.
Additionally, she added, “something that has emerged in the last 4-5 years is that social isolation and a lack of a social network can also be disease triggers. The incidence of Alzheimer’s in the Western situation has decreased, according to studies like FINGERS that focused on such modifiable lifestyle factors.
What is the scenario for India?
According to the World Alzheimer’s Report, 2021, just 1 in 10 dementia patients in India receive any sort of diagnosis, care, or treatment for the condition. Combating the disease is complicated by a lack of awareness. “The majority of our population incorrectly views it as a natural part of ageing. Medical assistance is only sought after the illness has progressed to an unmanageable point, such as when patients begin to wet their clothes without being aware of it. Unfortunately, the majority of patients visit us quite late, according to Dr. Tripathi.
In India, the incidence of dementia is predicted to rise in the next decades due to rising rates of diabetes, hypertension, and obesity. India might experience a 197 percent increase in dementia cases, including Alzheimer’s, from 3.84 million cases in 2019 to 11.44 million cases by 2050, according to the Global Burden of Disease report published this year in The Lancet.
Edited by Prakriti Arora




