
The long-awaited vaccine of Russia, Sputnik V has reportedly hit a roadblock in the manufacturing of the second dose of its vaccine in India. Several sources in the government have confirmed that the Russian vaccine developer is facing hurdles over manufacturing with the production of the second component (AD5) of the adenovirus vector-based vaccine. Government sources have also informed that a group of Russian scientists visited India for more than a month to try and find out the technical glitches that were being faced by domestic manufacturers while producing AD5.
According to experts. The second component of the vaccine is difficult to manufacture because of its pattern as an adenovirus vector-based vaccine. So what makes Sputnik V different from other vaccines? Well, the Russian vaccine unlike the others which use the same composition of doses for both the shots, Sputnik V uses two separate components in its vaccine. It uses two types of adenovirus vectors: rAd26 for the first dose and rAd5 for the second.
The main vaccine is Ad26 and after 3 weeks or 21 days, a booster dose is given which is rAd5. According to experts, the production of the second component is extremely time-consuming because the quality of the virus that can be produced in cell culture is considerably low. Consequently, if the yield is low, the quantity of the doses produced will be less.
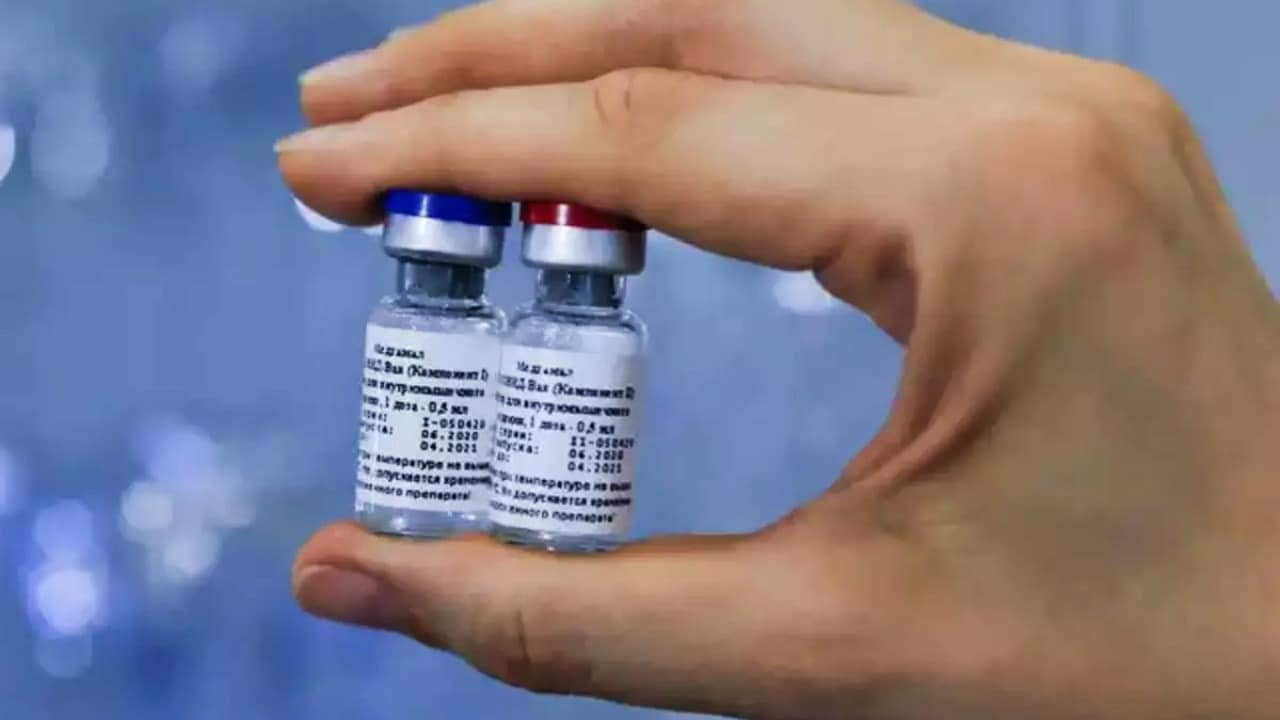
Sputnik V will impact India’s vaccination target
The roadblock hit by the Sputnik V producers has negatively impacted the availability of shots for India’s vaccination goal targeted by the government to immunize all above 18 years by the end of December 2021. The central government has filed an affidavit to the Supreme Court on vaccine doses that presented an estimation of expected doses from the Russian vaccine maker was set at 10 crores between August and December 2021.
This estimate was already lowered by the government from an earlier projection of 15.6 crore Sputnik V shots for August-December 2021. The government’s expectation was for 216 crore doses till December end. But in a press meeting held on July 2, Niti Aayog’s member (health) VK Paul announced that the government’s target for 216 doses is extremely hopeful which was made depending on the vaccine maker’s commitments.
According to the data provided on the Co-Win platform, Sputnik V have administered 4,44,638 doses in India to date. The second dose of the Russian vaccine is slated to be introduced in selected hospitals and vaccination centres in India in the coming days.
India’s dependence on imports

Presently, the Russian shots distributed in India has been imported from its mainland. According to V Muraleedharan, Minister of State, Ministry of External Affairs, India has already imported 31.5 lakh units of Sputnik’s first component and 4.5 lakh units of its second component doses will date.
Dr Reddy’s Laboratories in India are responsible for importing the doses of the Sputnik V vaccine. According to a company spokesperson for Dr Reddy’s Laboratories, they have already received 31.5 lakh Sputnik V doses of the first component and 4.5 lakh doses of the second component. The spokesperson also confirmed that the company is working closely with their six partners in the country for manufacturing preparedness.
The official also confirmed that the pilot programme that their company introduced in May 2021 has since witnessed a slow yet steady commercial increase all over the country. Dr Reddy’s have joined hands with several important hospitals all through the country and also set up their cold chain infrastructure across more than 300 places because the Russian vaccine requires -18 degree Celsius temperature. The Russian shot has been administered across 80 cities in India with more than 2.5 lakh people vaccinated to date.
What was the idea behind Sputnik Light?

Dr Reddy’s Laboratories put forward a proposal to the drug regulator of India on June 20 for an Emergency Use Authorization (EUA) or market authorization and a protocol for starting Phase 3 of the clinical trial for Sputnik Light. It is a single dose of the vaccine.
Unfortunately, even the single-dose shot proposal was obstructed by a regulatory hurdle. The SEC panel of India which is under the drug regulator restricted from permitting the Indian pharmacy company for a EUA. Some of the excerpts of the meeting cited the following:
“After detailed discussion, the committee suggested that the company should put forward the safety, efficacy and immunogenicity data from the phase 3 clinical trials of the Sputnik Light vaccine that is already being carried out in Russia for considering the proposal. Further, as the safety and immunogenicity data of dose one has already been generated in the country in another trial, there has been inadequate data and justification in conduction a separate similar clinical trial.”
Timeline of Sputnik V in India

The Russian vaccine was expected to begin producing a large number of doses in the month of July. The timeline has now been postponed to August. In India, the Russian Direct Investment Fund (RDIF) has manufacturing agreements with the Serum Institute of India, Hetero Group of Companies, Gland Pharma, Panacea Biotech, Virchow Biotech, Stelis Biopharma, and Morepen. It is expected that Dr Reddy’s will import 25 crore doses of Sputnik V for India from Russia under the deal.
Presently, a technology transfer for the manufacturing of Sputnik V in the country with a partnership of Serum Institute of India (SII), the RDIF informed that the first lot of the vaccines is projected to be dispatched from the production units in September. In a statement, the RDIF said that as a part of the technology transfer process, SII has already collected the cell and vector samples from the Gamaleya Centre. The statement also confirmed that the cultivation process has already kick-started with the approval of the import by the Drug Controller General of India.
According to the data published in The Lancet, Sputnik V has shown the efficacy of 91.6 per cent after the trials of Phase 3. On the other hand, Sputnik Light has shown an efficacy level of 80 per cent which according to the RDIF, is greater than that of many two-dose vaccines.
Government of Kerala in talks with Russia
The government of Kerala has initiated talks with Russia for setting up a Sputnik V vaccine production plant in the state as confirmed by the state’s government officials. According to Industries Minister P Rajeev, the state has allotted 10 acres of land for this project already. The Russian vaccine which is developed by Gamaleya National Research Institute of Epidemiology and Microbiology is one among the four Covid-19 vaccines accepted by India.
P Rajeev in a press meeting informed that the talks between Kerala and Russia had started long back when there was a massive shortage of vaccines. Initial discussions are in the process right now and discussions are focused on the terms and conditions of both sides. The chief secretary of the state and the industries officials are in conversation with the Russian officials, counsel general and direct investment board. Both parties are expected to sign the deal within a few days.
P Rajeev also mentioned that such a move by the Kerala government will immensely benefit the people of the state. Earlier, the Chief Minister of Kerala Pinarayi Vijayan had asked state officials to give suggestions to companies ready to produce Covid-19 vaccines in the state. The government had charged Kerala State Drugs and Pharmaceuticals Ltd (KSDP) and a committee of experts to speed up the process. The government had decided to allot the land in Thonakkal Life Science Park for setting up the production unit for the Russian vaccine.
Edited by Aishwarya Ingle




